With your project goals in mind, our scientists design screening assays to help ensure a successful hit identification campaign. We prioritise miniaturisation without compromising assay quality or reproducibility. We consider your desired mode-of-action — whether allosteric or orthosteric, fast or slow off-rate compounds — during assay development. To achieve in vitro conditions that best reflect physiological reality, we carefully characterise enzyme binding sites, substrates and co-factors.

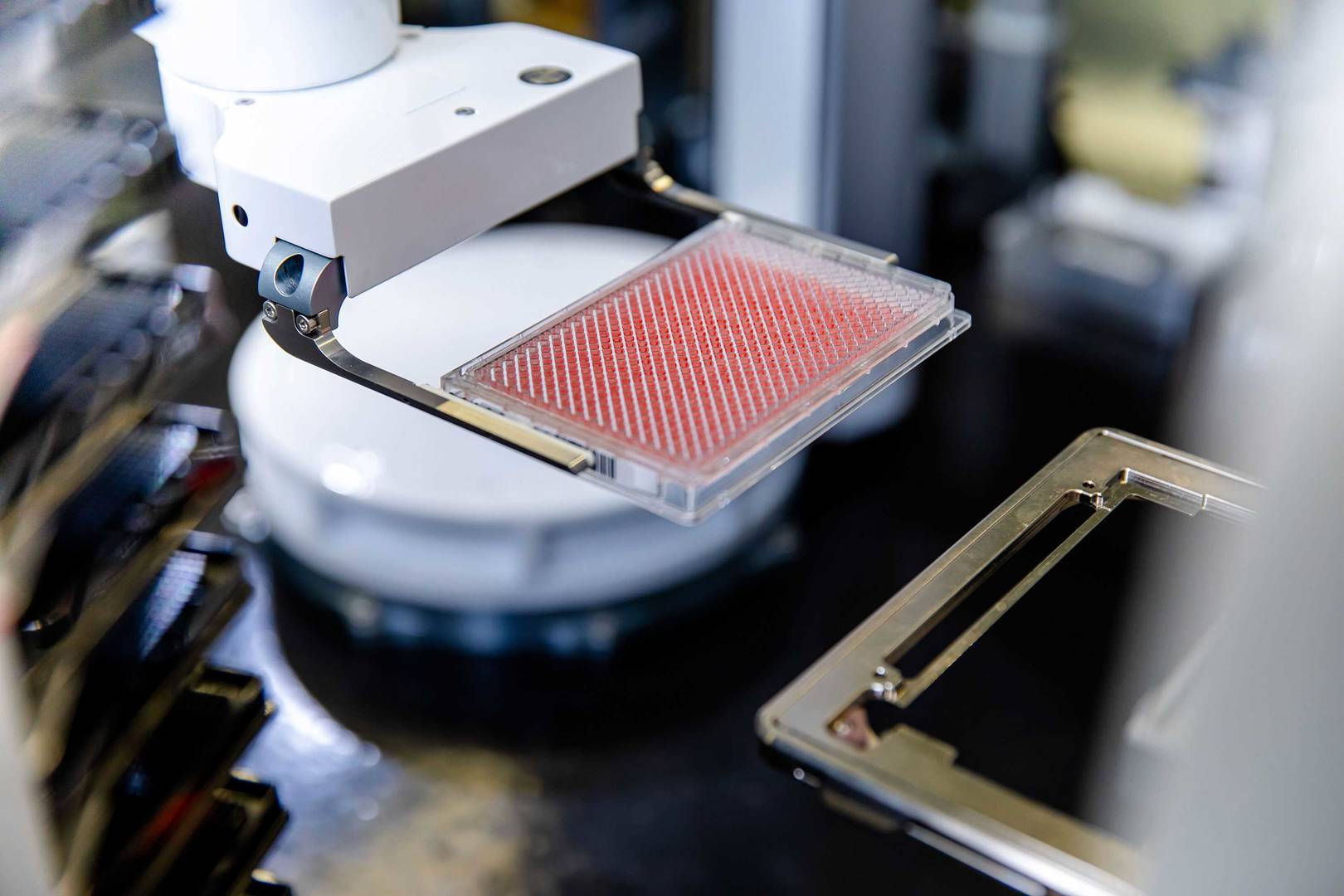
Our comprehensive high-throughput screening (HTS) services support you with the identification of your hit molecules.
LEARN MORE
Discover how we can support your project with our protein production solutions.
learn more