Our specialised assays are designed to help you refine hit lists and guide lead finding and optimisation, to provide a deeper understanding of compound behaviour in vitro and, ultimately, in vivo. Our team of screeners, protein scientists, structural biologists and chemists collaborates closely with you to deliver comprehensive insights that support your decision-making process, from off-target assays and species selectivity assays to comparative analyses of cell-based high-content screening, biophysical assays and biochemical data.


Our biophysical platform can support you with your lead identification and optimisation efforts.
learn more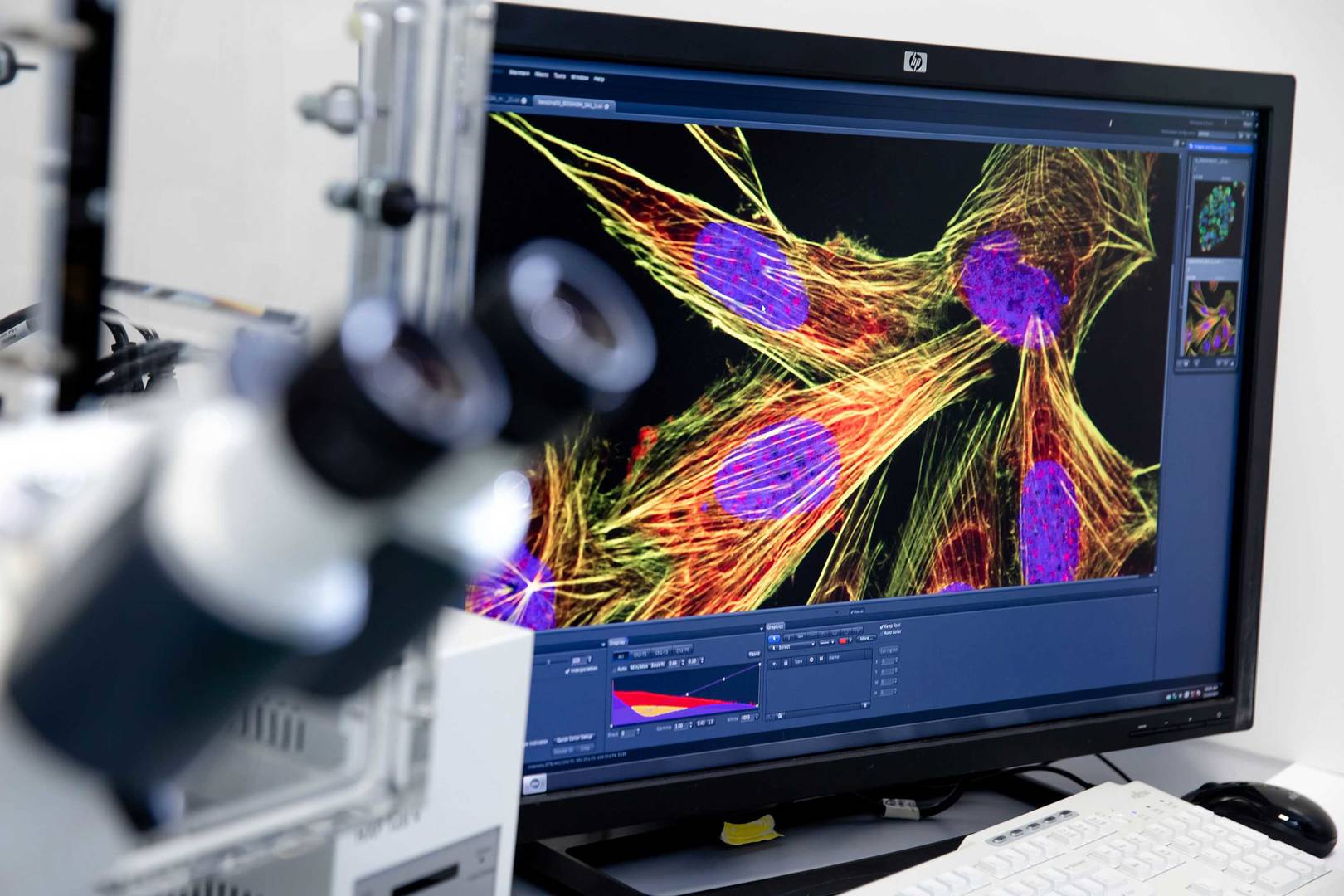
Our high content assay services provide precise and comprehensive cellular analysis.
learn more